Neuramis®
Global Hyaluronic acid filler, Neuramis®
Neuramis® is a high-quality hyaluronic acid filler manufactured with Medytox’s own technology.
Neuramis® is highly effective for skin rejuvenation, wrinkle correction and volume augmentation.

How does Neuramis® fill?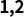
Hyaluronic acid (HA) fillers are the most common and non-permanent injectables.
Neuramis® could reduce facial wrinkles and restore facial volume.
It would be practical to use the different products considering the injection depth in skin.
 Example of Injection depth in Neuramis® series
Example of Injection depth in Neuramis® series 
Why Neuramis®?
1. Neuramis® is Safe²
1) High-quality of raw material
Neuramis® is manufactured using a high-quality hyaluronic acid that is registered in the Drug Master Files(DMFs) of FDA and certified by the European Directorate for the Quality of Medicines (EDQM). Neuramis® raw materials comply with even higher standards than required by the European Pharmacopoeia (Ph. Eur) to enhance safety.

 Management standard of HA raw materials
Management standard of HA raw materials 
| Category | Ph. Eur | Neuramis® |
|---|---|---|
| Residual protein | Maximum 0.3% or 0.1% | Less than 0.1% |
| Endotoxin | <0.5 or 0.05IU/mg | <0.04IU/mg |
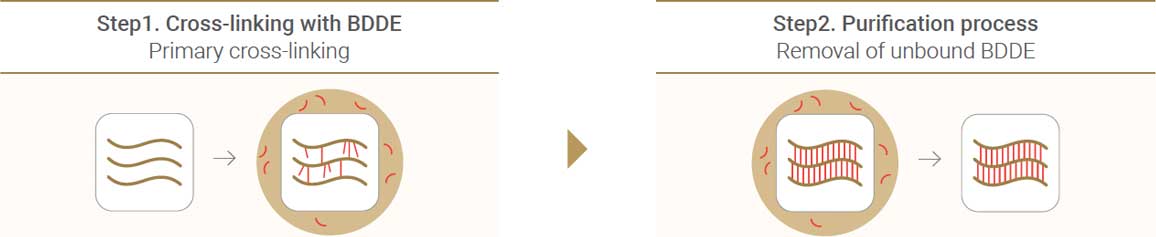
2) Improved Refining Process with SHAPE technology
SHAPE(Stabilized Hyaluronic Acid and Purification Enhancement) technology is the exclusive technology of Neuramis®. It stabilizes cross-linking and enhances the purification process. The Neuramis® series minimizes the amount of residual BDDE through a lengthy natural dialysis process.
 2 Step of cross-linking
2 Step of cross-linking 
3) In-house quality-control
The general industry standard for the amount of residual BDDE is less than 2 ppm; however, Medytox only sells products after complying with rigorous in-house quality-control standards which require products with non-detectable level of BDDE. (In-house equipment specification)
BDDE Standard solution
Neuramis® Series
4) Proven Safety
A comparative study on injections between Neuramis® Deep Lidocaine and Product A into nasolabial folds reported fewer adverse events from with Neuramis® Deep Lidocaine.
Number of Treatment-related adverse event
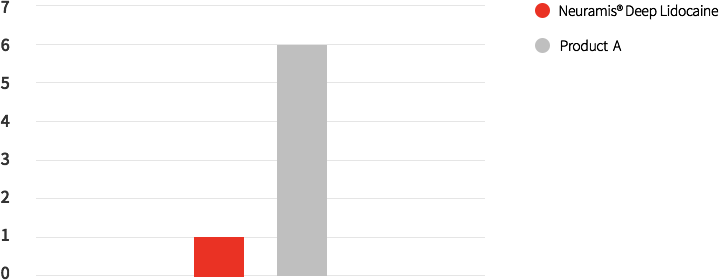
2. Neuramis® is Effective
1) Outstanding cohesiveness
Despite pressure from surrounding tissues, Neuramis® maintains its original shape after injection thanks to outstanding cohesiveness between the particles.
 Cohesiveness Test
Cohesiveness Test 
Neuramis® Deep

Biphasic

Other Monophasic

Video : Cohesiveness test
1) Neuramis® is not spread in the water and stayed in shape compared with Biphasic fillers.
2) Neuramis® can maintain its form and shape in the body.
2) Outstanding skin integration
Neuramis® shows outstanding skin integration which blends well with tissue around the skin after injection, giving a natural look.
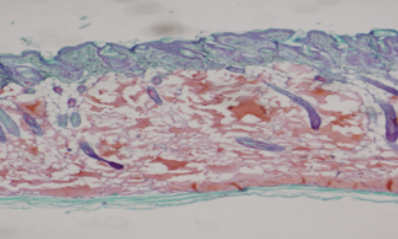
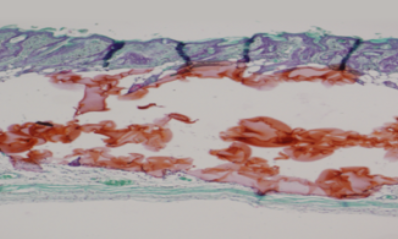
 Histologic images after filler injection
Histologic images after filler injection 
Non-injected
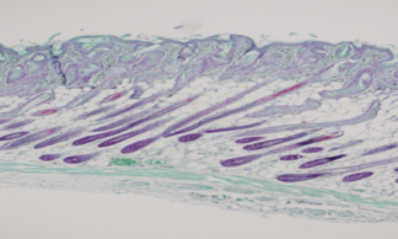
Neuramis® Deep
Biphasic
3) Proven efficacy
In a comparative study on injections between Neuramis® Deep Lidocaine and Product A, the nasolabial folds receiving the Neuramis® product showed great improvement and satisfaction.
<Efficacy Assessment>
WSRS Score by three Independent expert
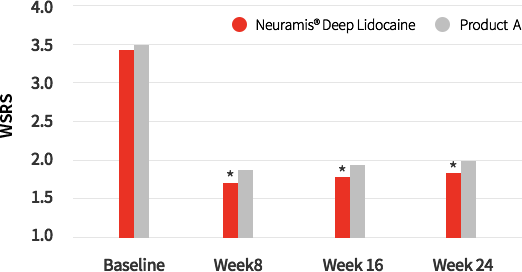
Fig1. Wrinkle Severity Rating Scale(WSRS) score of Neuramis® and control group evaluated with photographic assessment by three independent expert panels.
<Satisfaction Assessment>
GAIS Score by blinded Investigators
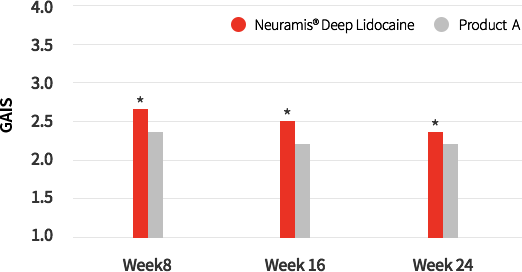
Fig2. Global Aesthetic Improvement Scale(GAIS) score for Neuramis® and control group evaluated with live assessment by blined investigators
* p<0.05, Difference between Neuramis® Deep Lidocaine and Product A.
Study Method
In a randomized, multicenter, double-blind, intra-individual comparison and controlled Phase III study on 60 subjects, injections of Neuramis® Deep Lidocaine and Product A were made to nasolabial folds of each subject to assess wrinkle improvement.
Efficacy Assessment
When photo evaluators assessed photos to measure changes in the Wrinkle Severity Rating Scale (WSRS), Neuramis® Deep
Lidocaine (1.64±0.74) showed great improvement in week 24 of the study.
(Neuramis® Deep Lidocaine : 1.64±0.74, Product A : 1.45±0.54)
Satisfaction Assessment
According to Global Aesthetic Improvement (GAIS) score evaluated by the investigators, Neuramis® Deep Lidocaine produced a high level of satisfaction in week 24 of the study. (Neuramis® Deep Lidocaine : 2.36±0.55, Product A : 2.00±0.55)
3. Neuramis® is a Global product
Neuramis® is a global product, registered in 36 countries with rapidly growing sales in more than 30 countries.
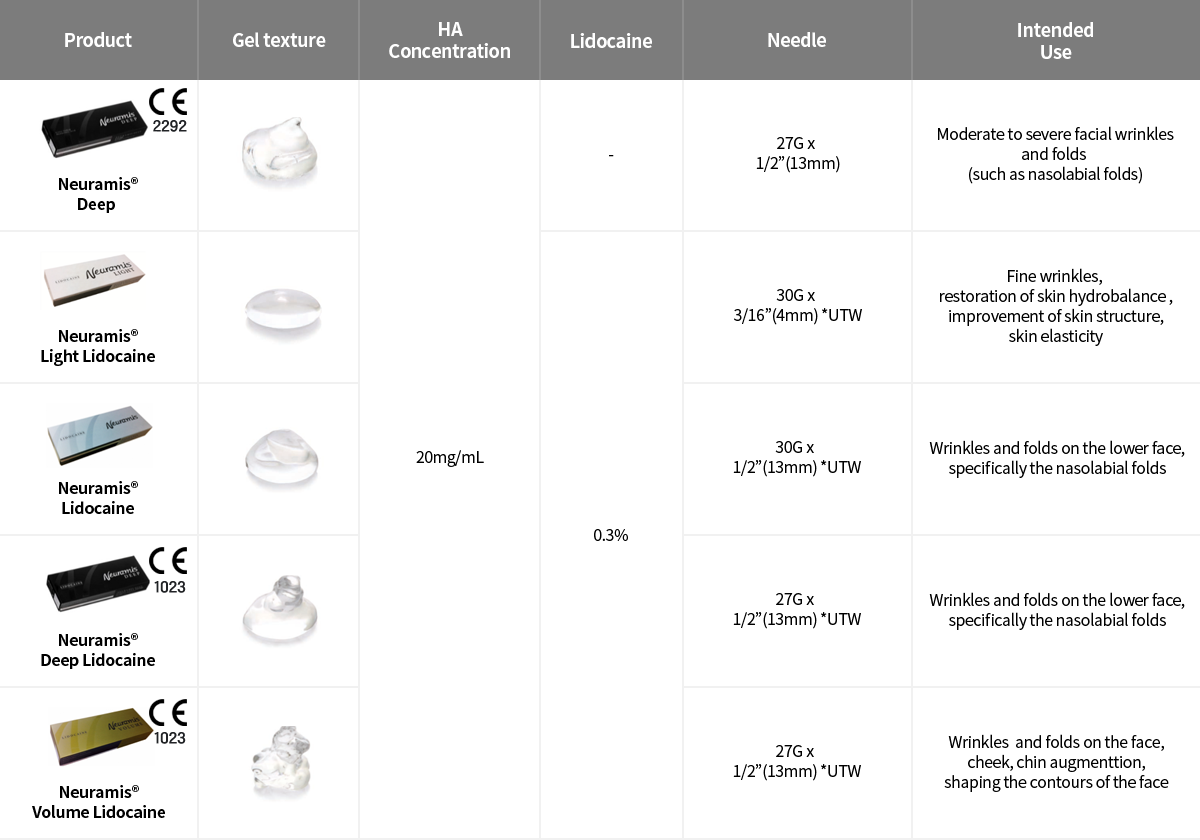
Product ranges
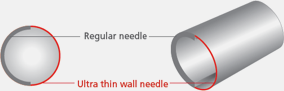
*UTW(Ultra Thin Wall needle)
The Ultra Thin Wall needle has a larger inner diameter compared to regular needles.
It improves flow rates and lowers extrusion force during injection.
Clinical studies
-
1. Neuramis® Deep
Changsik Pak et al. A Phase III, Randomized, Multi-Center, Double-Masked, Matched-Pairs, Active-Controlled Trial to Compare the Efficacy and Safety between Neuramis Deep and Restylane in the Correction of Nasolabial Folds, Arch Plast Surg. 2015 Nov;42(6):721-8.
-
2. Neuramis® Deep Lidocaine
Hong Jin Joo et al. A Randomized Clinical Trial to Evaluate the Efficacy and Safety of Lidocaine-Containing Monophasic Hyaluronic Acid Filler for Nasolabial Folds, Plast Reconstr Surg. 2016 Mar;137(3):799-808.
Neuramis® information
- Fredric S Brandt et al. Hyaluronic acid gel fillers in the management of facial aging, Clin Interv Aging. 2008 Mar; 3(1): 153–159
- Data on file
- Hong jin joo et al. Randomized Clinical Trial to Evaluate the Efficacy and Safety of Lidocaine-Containing Monophasic Hyaluronic Acid Filler for Nasolabial Folds, Plast Reconstr Surg. 2016 Mar;137(3):799-808














